Case of the Month: False Positive HIV Viral Loads
June 27, National HIV Testing Day, is an opportunity for patients and clinicians alike to play a pivotal role by promoting widespread HIV testing. In recognition of the importance of testing, the Clinician Consultation Center is sharing a testing-related case from its HIV/AIDS Management Consultation Service, which celebrates its 25th birthday this year!
Case Summary
A 40-year-old female presented to the caller with one week of flu-like symptoms. The patient also reported condomless intercourse with her long-term, healthy male partner approximately two weeks prior to the onset of symptoms. The patient had had a negative HIV test six months ago. Due to concern for possible acute HIV, HIV testing was conducted (using a 4th generation HIV Ag/Ab assay), and a separate specimen for HIV RNA testing was drawn and sent at the same time. Results from the 4th generation HIV test were negative; however, the viral load was reported as 6780 copies/mL. Testing was repeated one week later (i.e. approximately four weeks after the last possible exposure) and results were a negative 4th generation HIV test and “undetectable” HIV RNA PCR. The patient had not started any antiretroviral medications after initial results, nor had she ever taken post-exposure prophylaxis (PEP) or pre-exposure prophylaxis (PrEP).
The caller asked whether acute HIV had been reasonably excluded, and whether the initial viral load result should be interpreted as a false positive.
CCC Consultant Response
The CCC consultant advised that acute HIV is unlikely in this case, and that the first HIV RNA result was likely a “false positive” viral load1. Acute HIV infection (AHI) is the initial phase of HIV infection occurring immediately after transmission and before seroconversion. Approximately 40-90% of patients with AHI will develop symptoms such as fever, lymphadenopathy, sore throat, rash, and myalgias/arthralgias. Diagnosis of AHI is often challenging, although an advantage of the currently-recommended 4th generation testing algorithm is that inclusion of p24 antigen capture now allows detection of HIV-1 infection before seroconversion (i.e. development of HIV antibodies). Levels of HIV viremia are typically very high in AHI, i.e., greater than 100,000 copies/mL.
Figure 1. Sequence of appearance of laboratory markers for HIV-1 infection
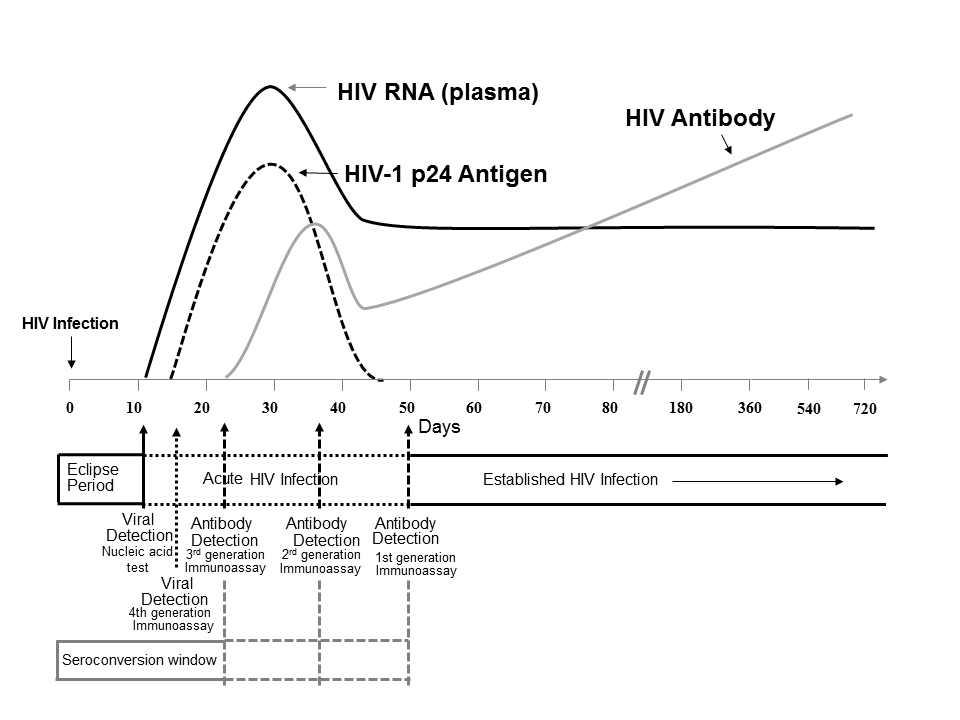
For this and similar cases, it may be helpful to refer to the CDC’s sequence of HIV-1 laboratory marker appearance (Figure 1) and to algorithms for AHI evaluation and diagnosis. Because the patient in this case was last potentially exposed to HIV four weeks ago, both HIV RNA and HIV-1 p24 antigen would be expected to be detectable at the time of repeat testing. Two 4th generation testing specimens collected one week apart yielded negative results, indicating no detectable p24 antigen in this patient2. These results, in addition to discrepant virologic test results including an initial RNA level < 10,000 copies/mL, suggest the initial HIV RNA was a false positive.
One key advantage of HIV RNA testing is its essentially 100% sensitivity at detecting HIV infection, particularly AHI. Additionally, studies from the early 2000s estimated specificity rates of approximately 95-98% for HIV-1 RNA PCR testing, noting slight differences across various detection methodologies. For example, branched DNA assays were slightly less specific than transcription-mediated amplification (95 vs. 98%). More recent investigations suggest specificity may be even higher than previously observed. Considered together, these performance characteristics of RNA testing may lead to highly uncommon but possible scenarios where results are reported as positive even though HIV-1 RNA is not actually present, resulting in false positives as illustrated by this case.
False positive viral load results have been reported in clinical trials as well as in clinical practice. Unfortunately, it remains largely unclear why false positive viral loads are seen in the general population. More common causes involve technical etiologies such as laboratory errors and contaminated testing devices. False positive PCRs have also been reported among patients that have undergone certain gene transfer therapies. For this reason, coupled with the importance of accurate and timely AHI diagnosis, comprehensive clinical and laboratory re-evaluation are imperative, alongside an understanding of the use and characteristics of various HIV tests.
1. After confirming with the caller there was no concern for HIV-2 infection↩
2. Note: sensitivity of p24 Ag detection is not consistent at RNA levels under ~50,000 copies/mL↩
References:
1. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. | Department of Health and Human Services.
2. Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations, June 27, 2014. | Centers for Disease Control and Prevention and Association of Public Health Laboratories.
3. Daar ES, Little S, Pitt J, et al. Diagnosis of primary HIV-1 infection. Los Angeles County Primary HIV Infection Recruitment Network. Ann Intern Med. 2001; 134(1):25-29.
4. Hecht FM, Busch MP, Rawal B, et al. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS. 2002; 16(8): 1119-1129.
5. Ren A, Louie B, Rauch L, et al. Screening and confirmation of human immunodeficiency virus type 1 infection solely by detection of RNA. J Med Microbiol. 2008; 57 (Pt 10): 1228-1233.
6. Rich JD, Merriman NA, Mylonakis E, et al. Misdiagnosis of HIV infection by HIV-1 plasma viral load testing: a case series. Ann Intern Med. 1999; 130(1): 37-9.
7. Havlichek DH Jr, Hage-Korban E. False-positive HIV diagnosis by HIV-1 plasma viral load testing. Ann Intern Med. 1999; 131(10): 794.
8. Stekler J, Maenza J, Stevens CE, et al. Screening for acute HIV infection: lessons learned. Clin Infect Dis. 2007; 44(3): 459-461.
9. De Ravin SS, Gray JT, Throm RE, et al. False-positive HIV PCR test following ex vivo lentiviral gene transfer treatment of X-linked severe combined immunodeficiency vector. Mol Ther. 2014; 22(2): 244-245.
Because CCC consultations are based on information provided by the caller or clinician accessing the online consultation center, without the benefit of a direct evaluation or examination of the patient, consultations are intended to be used as a guide. They do not constitute medical advice and are not to serve as a substitute for medical judgment. This Case of the Month includes consultation based on the most up-to-date evidence at the time of its publication. To learn about current recommendations, please call one of our clinical consultation lines.
 University of California, San Francisco |
University of California, San Francisco |
